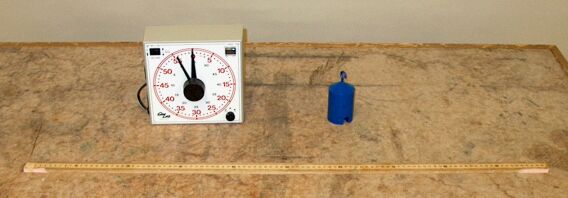

This page shows a clock, a 1-kg mass and a meter stick, which represent the three most common physical quantities we use in characterizing things in physical terms – time, mass and length – and their SI (Système International) units, the second, the kilogram and the meter.
When we observe the workings of a physical system in order to formulate expressions to describe its behavior, we must be able to measure the relevant properties of the system, so as to quantify the behavior in which we are interested. If we were to do this by ourselves, it would be important to establish some system of units in order to understand what we were doing. If we then wished to describe our observations to other people, either we would have to explain our system of units each time we did this, or we would have to use some kind of standard system of units, which, by convention, anyone could understand. Because of the large number of physical properties we can measure, and the various relationships among them, however we choose to build our system, we will end up with a certain number of units that are basic, or base units, which we can combine to form a variety of derived units. For example, dividing the base unit of length by the base unit of time gives us the derived unit of velocity, or, for an example of a derived unit that has its own name, the combination (kg·m/s2) gives us the unit of force, called the newton, abbreviated as N. In developing our system, we choose the smallest number of quantities necessary to describe physics completely in the simplest way possible. To establish a standard system that everyone could understand and use, on May 20, 1875, seventeen countries (Argentina, Austria-Hungary, Belgium, Brazil, Denmark, France, Germany, Italy, Peru, Portugal, Russia, Spain, Sweden and Norway, Switzerland, Turkey, The United States of America, and Venezuela) signed a treaty known as the Metre Convention, which created the International Bureau of Weights and Measures (Bureau International des Poids et Mesures, BIPM), located in Sèvres, France. (Now there are 59 member states and 42 associate states and economies. You can also find interesting information on the Metre Convention here.) This treaty was prepared by the International Metre Commission, which had been established in 1870 in Paris. May 20 is now celebrated as World Metrology Day. The BIPM was established under the authority of the General Conference on Weights and Measures (Conférence Générale des Poids et Mesures, CGPM), and the supervision of the International Committee for Weights and Measures (Comité international des poids et mesures, CIPM). In 1960, the 11th General Conference on Weights and Measures established the International System of Units, or SI (from the French Système International d’Unités). They chose the following six quantities as their base units:
Quantity Name Symbol Length meter m Mass kilogram kg Time second s Electric current ampere A Thermodynamic temperature kelvin K* Luminous intensity candela cd
*The original unit was the degree Kelvin. In 1967, the 13th CGPM changed this to the kelvin. In 1971, the 14th CGPM added a unit for amount of substance, the mole (abbreviated mol), to the six base units listed above, bringing the number of SI base units to seven.
Sometimes the base unit is either too large or too small to provide a convenient measure for a particular physical quantity. For example, the meter, by itself, is far too large to describe the distance between atoms in a molecule (on the order of about 10-10 m), and far too small to use as a measure of the distance from the earth to the sun (on the order of 1011 m). The CGPM thus adopted the following prefixes by which to multiply units:
Factor Name Symbol Factor Name Symbol 101 deka da 10-1 deci d 102 hecto h 10-2 centi c 103 kilo k 10-3 milli m 106 mega M 10-6 micro μ 109 giga G 10-9 nano n 1012 tera T 10-12 pico p 1015 peta P 10-15 femto f 1018 exa E 10-18 atto a 1021 zetta Z 10-21 zepto z 1024 yotta Y 10-24 yocto y The kilogram is the only base unit that itself contains a prefix. Prefixes may not be combined, so if one has 10-9 kg, this equals 10-6 g or 1 μg (microgram), not 1 nkg (nanokilogram).
The meter
The original international standard of length was a platinum-iridium bar, with a fine line engraved near each end, kept at the International Bureau of Weights and Measures. The distance between the two lines, one meter, was supposed to equal one ten-millionth the distance from the north pole to the equator along the meridian line that runs through Paris. (It is actually slightly different from this value because of a miscalculation of the flattening of the earth due to rotation, and thus an error in the determination of the length of this quarter meridian.) In 1960, the 11th General Conference on Weights and Measures redefined the standard meter as 1,650,763.73 wavelengths of the (orange-red) light emitted by atoms of Kr86 when they make the transition from the 5d5 state to the 2p10 state. (This is a transition from a 3D1 state to a 3P1 state, involving the dropping of an electron from a 6d orbital to a 5p orbital. In these term symbols, the left superscript denotes the electron spin multiplicity, 2S + 1, where S is the total electron spin for the state. The italic capital letter, S, P, D, F, etc., denotes the total orbital angular momentum of the valence electron(s), 0, 1, 2, 3, etc. The right subscript denotes J, the vector sum of the spin and orbital angular momenta, L + S. J has the range L + S, L + S - 1, L + S - 2, . . . , |L - S|.) In 1983, the CGPM redefined the meter as the distance light travels in vacuum in a time interval of 1/299,792,458 second. (This fixes the speed of light at exactly 299,792,458 meters/second.)
The kilogram
At the end of the 18th century, a kilogram was the mass of a cubic decimeter of water. In 1889, the first CGPM defined the kilogram as the mass of a platinum-iridium prototype, kept at the BIPM. Until fairly recently, it was the last unit remaining whose basis was still a physical prototype. In November, 2018, CGPM agreed that the kilogram should be defined in terms of Planck’s constant, and on May 20, 2019, this new definition was adopted. (See demonstration 88.10 – Planck’s constant demonstration.) Planck’s constant, which gives the relationship between the energy of electromagnetic radiation and the frequency of its oscillation, has the units of kg·m2/s. Once the meter and second are defined, precise determination of Planck’s constant gives the basis for the standard kilogram. In June, 2017, NIST (National Institute of Standards and Technology, formerly National Bureau of Standards) measured Planck’s constant to be 6.626069934 × 10-34 kg·m2/s, with an uncertainty of 13 parts per billion. You can read about this measurement here, and about the change to the new definition here.
The second
In measuring time for various events, we may need to know at what time of day they occur, so we can know in what order they happened, or we may need to know the time intervals over which they occurred (how long they took). The rotation of the earth, which determines the length of the day, is the basis of our civil time standard. One (mean solar) second is defined as 1/86,400 of a (mean solar) day. This was actually the original basis for the SI unit, the exact definition of “mean solar day” being left to astronomical theory. Measurement showed that the theory could not account for irregularities in the earth’s rotation, and this standard was thus not sufficiently accurate. The International Astronomical Union proposed a definition based on the tropical year (the time from one vernal equinox to the next), which the 11th CGPM adopted. By that time, work had begun on an atomic standard, and in 1967 the 13th CGPM redefined the second as the duration of 9,162,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the cesium-133 atom. (The splitting of the ground state into these two levels is due to the coupling of the spin of the valence electron with the nuclear spin.)
The links above, and the various links on the pages to which they lead, provide much interesting information about the International System of Units, International Bureau of Weights and Measures, General Conference on Weights and Measures, International Committee for Weights and Measures, the units themselves, and the history of their definition. In addition, you can find information on the SI units and their history at the NIST website. For information on the fundamental physical constants, you can go to a different page by NIST, or to the web page of the Committee on Data of the International Council for Science (CODATA).
For a humorous note, someone had a hysterically funny letter regarding systems of units published in Nature, which the editor of American Journal of Physics reprinted here.
References:
1) Resnick, Robert and Halliday, David. Physics, Part One, Third Edition (New York: John Wiley and Sons, 1977), ch. 1.
2) Pauling, Linus. General Chemistry, Third Edition (New York: Dover Publictions, Inc., 1970), p. 3.