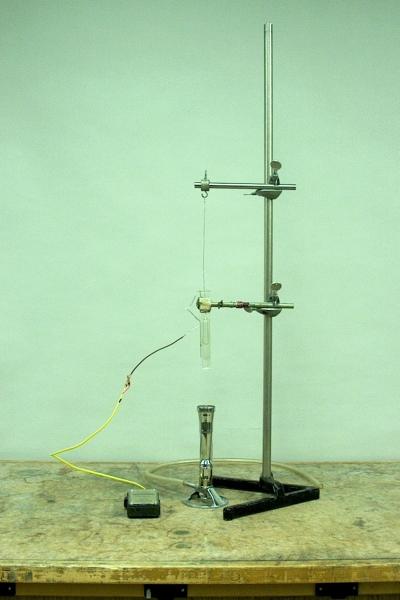

The sealed thick-walled glass tube in the apparatus above contains diethyl ether. At room temperature, the tube contains both the gaseous and liquid phases in equilibrium with each other, the liquid occupying about 40% of the volume of the tube. The tube is suspended (it has a hook on one end by which to do so) in a test tube filled with mineral oil. A thermocouple immersed next to the critical point tube allows you to monitor the temperature as you heat the apparatus by means of the Méker burner. (You must use the apparatus on the main demonstration table, which has a natural gas tap.) Since the volume is held constant, as you heat the tube, both the temperature and pressure increase until the ether reaches its critical point.
In the (p-T) phase diagram for any substance that can exist in a liquid or gaseous state, the region in which the substance exists as a liquid abuts the region in which it exists as a vapor. Along the boundary between these two regions, the liquid and vapor phases coexist in equilibrium with each other. Raising the temperature or lowering the pressure vaporizes the liquid, and lowering the temperature or raising the pressure condenses the vapor. At some temperature and pressure, however, this line ends. Past this point, the liquid and vapor phases become indistinguishable. This point is called the critical point. For ether, the CRC Handbook of Chemistry and Physics (51st Edition, 1970-1971) gives the critical point as 193.4 °C and 35.6 atm. Above the critical point, it is impossible to condense the vapor merely by increasing the pressure; one must lower the temperature as well. It is possible, starting below the critical point, to heat a gas at constant pressure, then isothermally compress it, and finally cool it at constant pressure, thus going around the critical point, making the density increase smoothly and continuously to take the gas into the liquid state without having it undergo the sudden transition of condensation.
The photographs below show how the demonstration looks:
This is how the tube looks at the start of the demonstration:
Note that there is liquid at the bottom, vapor at the top, and the meniscus is concave.
When the temperature reaches 204 °C, the meniscus begins to fade, and by 210 °C, it has totally disappeared. There is now one continuous phase inside the tube.
CAUTION: ONCE THE TUBE REACHES THIS POINT, IMMEDIATELY REMOVE THE BURNER!
When the tube is allowed to cool, the ether passes back through the critical point, at which time a very interesting phenomenon occurs. At the critical point, the thermodynamics of the system becomes very interesting. Here, (∂p/∂v)T = 0, so the isothermal compressibility (-1/v(∂v/∂p)T) becomes indefinitely large. This implies that isothermal compression or expansion entails vanishingly little work. We can imagine the fluid as being divided into tiny parcels, each of which can exchange energy and matter with its surroundings. Since (∂p/∂v)T = 0, we would expect fluctuations in the densities of these parcels to be large. Since the volume and the amount of material are fixed, so is the average density. For any parcel having above-average density, then, some other parcel must have below-average density, and these fluctuations are thus coupled. These density fluctuations also give rise to fluctuations in the refractive index of the fluid, which cause light passing through the fluid to be scattered. At the critical point, these fluctuations increase to where the scattering is so great that the fluid appears milky and opaque. This phenomenon is known as critical opalescence.
In a cell that is uniformly heated and has a good thermostat, if the substance is held at its critical point or within a few tens of millikelvin of it, the entire cell becomes cloudy and dark, and the critical opalescence exhibits a brownish color. For examples, please see references here and here. In this demonstration, it is not possible to hold the entire cell at the critical point, nor is it possible to hold any part of it there for very long. (In fact, since we cannot control the pressure, unless the amount of ether in the tube is exactly right, it may not pass exactly through the critical point.) As a result, we see light scattering only from the region where the single phase is beginning to separate into two phases, as in the photograph at right.
Once the tube cools below the critical point, the meniscus reappears as in the top photograph.
References:
1) Levine, Ira N. Physical Chemistry (New York: McGraw-Hill, Inc., 1978), pp. 169-173.
2) Berry, R. Stephen; Rice, Stuart A. and Ross, John. Physical Chemistry (New York: John Wiley and Sons, 1980), pp. 876-878.
3) Resnick, Robert and Halliday, David. Physics, Part One, Third Edition (New York: John Wiley and Sons, 1977), p. 534.