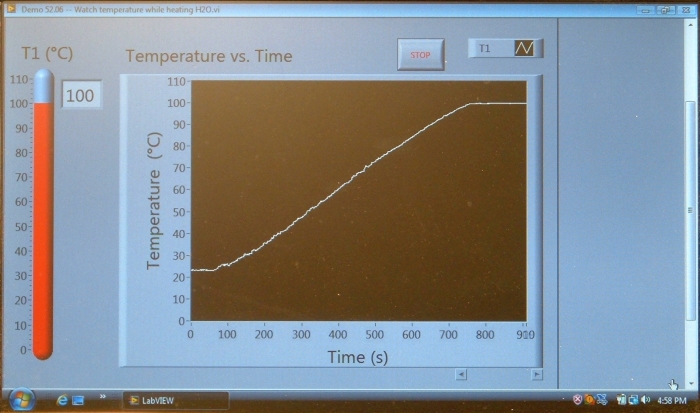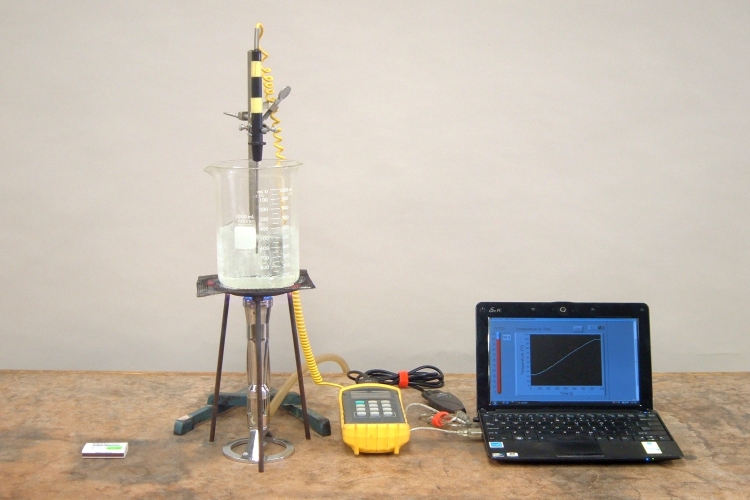
A thermocouple probe sits in a beaker of water that is at room temperature. The thermocouple is connected to a controller, which is connected to a netbook that shows a readout of the temperature, and a plot of the temperature over time. The netbook can be connected to the data projector so that the class can see its display. When you light the burner underneath the beaker, the water heats up, and its temperature begins to rise steadily until it begins to boil, at which point the temperature stays at 100 °C. The temperature readout does not rise above 100 °C, and the plot flattens. No matter how much additional heat you add, as long as there is liquid water in the beaker, the temperature remains at 100 °C.
The water in the beaker is in equilibrium with its vapor above. That is, at any given time, an equal number of molecules is going from the liquid phase into the vapor phase, as is going from the vapor phase to the liquid phase. At room temperature, the pressure of the vapor, that is the vapor pressure of the water, is about 17.5 torr (about 0.338 psi, or 0.0230 atm). This pressure depends on the average kinetic energy of the molecules in the liquid, which increases with temperature. Thus, as you increase the temperature of the liquid water, its vapor pressure increases until it equals the pressure of the air above the beaker, which is one atmosphere. At this point, bubbles of vapor form throughout the liquid and rise to the top; the water boils. The temperature at which this happens is 100 °C, the normal boiling point of water.
Until the temperature of the water reaches the boiling point, the heat that you add to to the water increases its internal energy, and thus its temperature. Once the temperature reaches the boiling point, however, any additional heat that you add goes into vaporizing the water. The energy necessary to transform water from the liquid phase to the vapor phase is called the heat of vaporization, and it equals 539 calories/gram (or 2,250 joules/gram, or 40,500 joules/mole). Once the temperature reaches the boiling point, as long as there is still liquid water, any additional heat that you add provides this heat of vaporization and vaporizes more liquid water. This continues until all the liquid has been vaporized, and until that happens, the temperature of the liquid remains at 100 °C.
Below is a photograph of the computer display, showing the temperature readout and graph after the water was heated from room temperature to the boiling point, and then held there for about 2-1/2 minutes. Note that once the temperature of the water reaches 100 °C, it stays there.
References:
1) Sears, Francis Weston and Zemansky, Mark W. College Physics, Third Edition (Reading, Massachusetts: Addison-Wesley Publishing Company, Inc., 1960), pp. 314-316, 371.