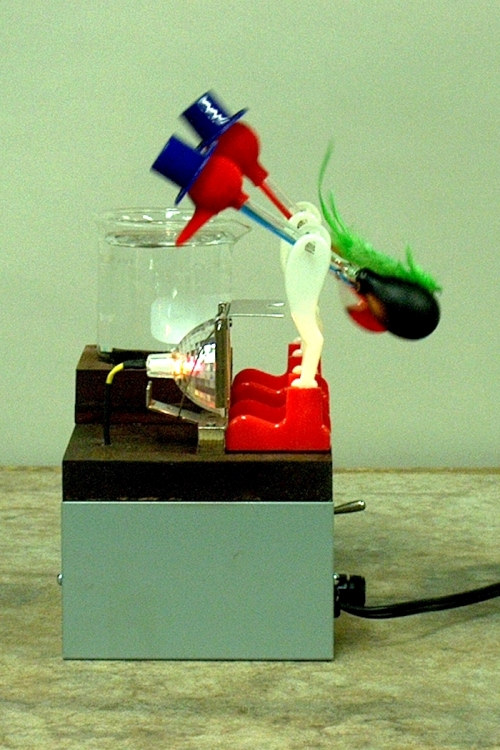

In the photograph above are two drinking birds, a popular novelty. The one at rear, which has a beaker of water in front of it, operates in the normal way. You first wet its head, then place it on its pivot, and it begins to work. The one in front, with its abdomen painted flat black, has a lamp in front of it, which you turn on by means of the switch that you can see protruding from the right side of the box in the photograph. After the lamp has been on for a few minutes, the bird begins to work.
The drinking bird comprises two glass bulbs connected by a glass tube. The top bulb, the bird’s head, is spherical, and is covered with flocking, which also covers the beak that is attached to it. The bird’s abdomen is elongated, and the tube that connects the head and the abdomen, extends into the adbomen, almost to the bottom. The apparatus is filled with methylene chloride (dichloromethane, CH2Cl2), which is a volatile liquid. (Its vapor pressure at room temperature (70°F/2 °C) is about 364 torr, or about 0.48 atmospheres. Its boiling point at atmospheric pressure is 40°C (104°F)) When the bird is filled, the residual gas is evacuated, so that when the bird is sealed, it contains liquid methylene chloride in equilibrium with its vapor. To operate the drinking bird, you wet the whole head, then set it on its stand in front of a glass (or in this case, a beaker), filled with water to a level where when the bird tips, the beak gets wet. The flocking on the head and beak absorb water, which keeps the head wet as the bird operates. At the beginning, the bird is upright, and all the liquid methylene chloride is in the abdomen. The water in the flocking on the head begins to evaporate. To do so, it must extract thermal energy from the head, and the head cools. (Water is also somewhat volatile, though much less so than methylene chloride, and has a vapor pressure, at 21°C, of about 19 torr, or about 0.02 atmospheres.) This reduces the pressure of the methylene chloride vapor in the head, and the pressure of the vapor in the abdomen becomes great enough to push the liquid up through the glass tube and into the head. This causes the center of mass of the bird to rise and move forward of the pivot, so that when the liquid progresses far enough into the head, the bird tilts forward, dipping the beak into the beaker of water. When this happens, the bottom end of the tube rises above the level of the liquid in the abdomen, and some of the vapor in the abdomen can now pass into the head, warming it and equalizing the pressure, and allowing the (somewhat cooler) liquid now to flow back into the abdomen, which becomes heavy enough to rotate the bird into its original, upright position. The head begins to cool again, and another cycle begins.
The drinking bird is, essentially, a heat engine, in which the hot reservoir is the abdomen, and the cold reservoir is the head. The working fluid is methylene chloride, and it is put through its cycle by transfer of heat between the abdomen and the head. In the bird as it operates normally, the temperature gradient between the hot and cold reservoir is maintained by evaporative cooling of the head. We can, however, operate the bird in a different way, which is illustrated by the bird in front, with the black-painted abdomen and the lamp. By means of the lamp, we heat the abdomen of the bird. This raises the vapor pressure in the abdomen, forcing the liquid up the tube into the head. As in the description above, when the liquid rises to the point where the center of mass of the bird is now in front of the pivot, the bird tips over, allowing some of the warmer vapor to flow into the head, and some of the (cooler) liquid to flow back into the abdomen. When enough liquid has flowed into the abdomen, the center of mass of the bird falls back below the pivot, and the bird rights itself. It is now ready to begin another cycle.
References:
1) Weast, Robert C., Editor. Handbook of Chemistry and Physics, 51st Edition ( Cleveland, Ohio: The Chemical Rubber Company, 1970), pp. C-367, D-143, D-146. (Boiling point of methylene chloride, vapor pressure of water at 21°C, vapor pressure of methlyene chloride at 21°C.)
Some interesting papers about the drinking bird:
1) Henry A. Bent and Harold J. Teague, The hydro-thermal-dynamical duck. Journal of college science teaching 8 (1) pp. 18-28 (September 1978).
2) Robert Menzer, The drinking bird – the heat engine that could. The physics teacher 31 pp. 126-7 (February 1993).
3) Lily M. Ng and Yvonne S. Ng, The thermodynamics of the drinking bird toy. Physics education 28 (5) pp. 320-4 (1993).
4) G.K. Vemulapalli, A discourse on the drinking bird. Journal of chemical education 67 (6) pp. 457-8 (June 1990).
5) Ralph Lorenz, Finite-time thermodynamics of an instrumented drinking bird toy. American journal of physics 74 (8) pp. 677-82 (August 2006).