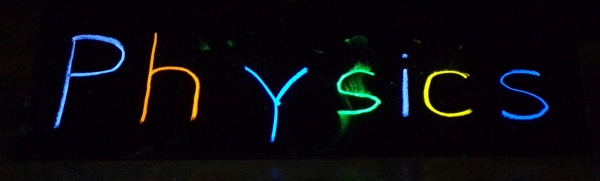At left in the photograph is an ultraviolet lamp. When you darken the room and illuminate the various objects on the table with it, the face of the detergent box and the outlines of the letters on it glow, the letters on the “Physics” sign glow in different colors, the Blak-Ray fluorescent chalk, with which the sign was made, glows in different colors, and the mineral samples glow in different colors, as shown below. A second lamp, shown at right, is also available. Please note that the UV emissions from both of these lamps are not safe to look at directly.
The UV lamp at left is a UVP Model UVG-54 Mineralight® lamp, which emits light whose wavelength is 254 nm. The lamp at right is a Spectroline Model MB-100 super high intensity black light lamp, which emits light whose wavelength is 365 nm. Please note that the UV emissions from both of these lamps are not safe to look at directly. The photographs above show the laundry detergent box, the “Physics” sign made with fluorescent chalk, and the Blak-Ray fluorescent chalk with which it was made, illuminated by the UVP Model UVG-54 Mineralight® lamp. Below are photographs of the mineral samples illuminated by each of these lamps.
Mineral samples illuminated with 254-nm UV light Mineral samples illuminated with 365-nm UV light These minerals, numbered, from left to right, one through five in the back row and six through 10 in the front row, are:
1) Willemite (Zn2SiO4)in calcite (CaCO3); fluoresces green under UV light.
2) Fluorite (CaF2); fluoresces blue under UV light.
3) Barite (BaSO4); fluoresces pink under UV light.
4) Autunite (Ca(UO2)2(PO4)2·10-12H2O) fluoresces yellow-green under UV light.
5) Sphalerite (ZnS); fluoresces red under UV light.
6) Wernerite (scapolite; mixture of Ca4(Si, Al)12O24(CO3=, SO4=) (marialite) and Na4(Al, Si)12O24Cl (meionite);
Fluoresces orange under UV light.
7) Calcite (CaCO3); fluoresces cream under UV light.
8) Resinous coal (C, with resin-containing particles or veins of vegetable matter); fluoresces yellow under UV light.
9) Onyx (sardonyx, SiO2, with impurities); fluoresces yellow under UV light.
10) Hackmanite (a variety of sodalite, Na8Al6Si6O24(2Cl-, S=); fluoresces pink under UV light.Demonstration 88.15 -- Spectra, shows the electronic excitation of atoms by their absorption of light, and the emission of light by electronically excited atoms (excited in an electrical discharge) as they relax to lower-energy states. That demonstration also has a fluorescent paddle, which allows you to show some of the lines that mercury emits in the ultraviolet. Just as atoms have an electronic structure in which an electron (a valence electron, in the transitions that we are discussing) can be excited from a particular energy level to a higher energy level by the absorption of light, or can relax from a high energy level to a lower one by emitting light, so do molecules. In order for this process to occur, the atom or molecule must have an electronic state whose energy is low enough that the energy of the incoming light equals the gap between it and the electronic ground state. For molecules, each electronic state has within it multiple vibrational states (and rotational states, which are greatly broadened unless the molecule is in the vapor phase), so that as long as the energy of the incoming light equals or exceeds the difference between the excited electronic state and the ground electronic state, there will be some level in the excited electronic state to which the light can excite the molecule. Unless the molecule is isolated, excess vibrational energy in the excited electronic state is quickly lost through collision (or to neighboring molecules in a solid). The electron then makes the transition from the excited electronic state to the ground electronic state, and the molecule relaxes to whatever vibrational levels in the ground state for which this transition is allowed, emitting light in the process. This type of emission is called fluorescence, and the time it takes for it to occur is typically on the order of 10-8 s. The light emitted is lower in energy, so of longer wavelength, than the light absorbed by the molecule. As this demonstration illustrates, molecules that have the appropriate electronic structure can absorb ultraviolet light, which is invisible, and by fluorescence emit visible light. Atoms can also undergo this process. Since atoms cannot rotate or vibrate, however, if they are isolated they emit light of the same wavelength as the light that they absorb (which must be only certain wavelengths in order for the atom to absorb it). This is known as resonance fluorescence. If they can lose energy via collision, or if they are bound within a crystal lattice to which they can lose energy, atoms can fluoresce by emitting light of longer wavelength than that of the absorbed light.
In addition to providing a tool that is quite useful in a variety of spectroscopic techniques, this phenomenon has several practical applications. In fluorescent lamps, the mercury vapor inside emits several discrete lines in the visible range, and some in the ultraviolet (see demonstration 88.15 -- Spectra). A phosphor coating on the inside of the lamp absorbs the ultraviolet and higher-energy visible light emitted by the mercury, and fluoresces to produce emission that covers more of the visible spectrum and extends into the red. Many laundry detergents contain organic compounds called optical brighteners. Such compounds are also used in making paper. By absorbing ultraviolet light and then emitting visible light along with the light that we would normally see reflected by either the clothing or the paper, these compounds make the clothing or paper appear brighter than they normally would. If you shine one of the ultraviolet lamps on a freshly laundered piece of clothing, it fluoresces with a blue or purple color, as does a piece of paper or business card so illuminated. This makes business cards useful for following ultraviolet laser beams, which would otherwise be invisible, to align the optics that steer them. Used in certain types of road signs, fluorescent materials absorb UV light from the sun and emit visible light, making the signs stand out. This is why sometimes, the orange signs that alert motorists to road work or other abnormal conditions, appear brighter than their surroundings. Fluorescent paint is often used to make objects easier to see, and fluorescent materials are used in some safety vests. Because they absorb ultraviolet light to emit visible light, these compounds are often called UV shifters.
The transitions described above are between electronic states that have the same spin multiplicity (2S + 1, where S is the total electron spin for the state); they are allowed transitions. For most molecules, the ground state is a singlet state (S = 0; 2S + 1 = 1), and so is the first excited electronic state. For some molecules, however, there is a state below the excited singlet state, for which the spin multiplicity is different. It is often a triplet state (S = 1; 2S + 1 = 3). Through a process called intersystem crossing, a molecule excited to the first excited singlet state can make a transition to a level that is equal in energy within this triplet state (or to an excited triplet state above it, from which it relaxes to the lower triplet state). Since the spin multiplicity of this state is different from that of the ground state, the transitions between it and the ground state are spin forbidden, and the triplet state is long lived, or metastable. These transitions do occur, though, and when the molecule relaxes from the triplet state to the ground state, it emits light, again of a lower energy (longer wavelength) than that which initially excited it. The lifetime of the metastable triplet state can be anywhere from about 10-3 seconds to hundreds of seconds or even longer. This type of emission is called phosphorescence. Atoms that have multiple valence electrons and thus states that have different spin multiplicities, can also undergo this process. Below are two photographs of glow-in-the-dark paracord, which is impregnated with a phosphorescent compound. The one at left was taken in normal room light, and the one at right was taken with the room darkened. (The paracord was merely allowed to sit under the fluorescent lights, and then the lights were turned off.) The paracord is not shown above, but is available for use with this demonstration.