My first proper internship was a summer spent at Rutgers University, under the supervision of Jing Li, who graciously let an inexperienced, incompetent, and danger-prone high schooler work with her then-postdoc, Debasis Banerjee. All images in this summary were produced in her lab.
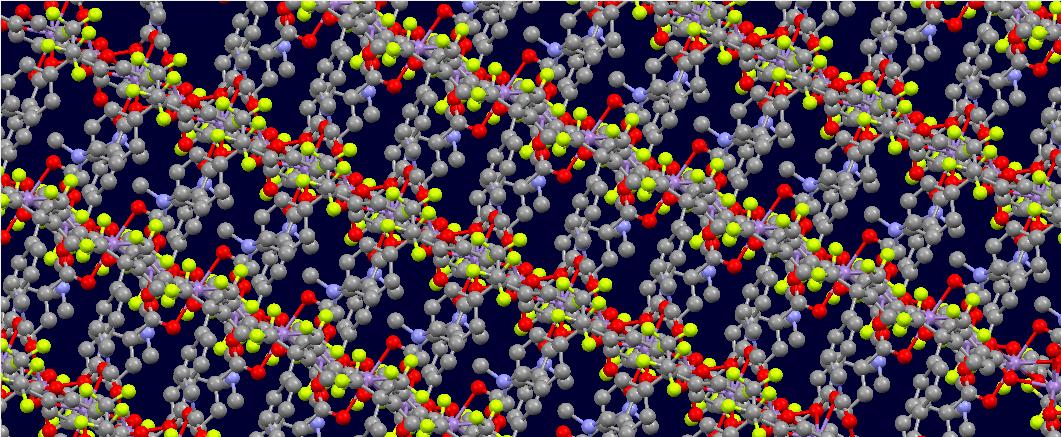
Metal-organic frameworks (MOFs) are porous crystals made of a metal center and an organic acid ligand. These compounds are interesting because they are some of the most porous materials known, are cheap to produce, and can be used for applications like CO2 capture and luminescence-based explosive detection. The creation process for an average MOF involves dissolving a metal salt in solution with an organic acid and a solvent and heating to evaporate some solvent and precipitate a crystal. Sometimes, though not always, the solvent can become part of the crystal structure, attaching to any remaining binding sites on the metal center.
As water typically reacts poorly with some reagents, other solvents were used like methanol and ethanol, though these both have a relatively low boiling points of 65°C and 78°C, respectively. Typically, we would use dimethylformamide (DMF) or its closely related cousins dimethylacetamide (DMA) and diethylformamide (DEF) in combination with an alcohol. These solvents would both become part of the lattice and become trapped in the pores, while the alcohol would evaporate. DMA and DEF were useful as, depending on whether a solvent binding to the lattice is desirable or not, their larger carbon side chains could sterically hinder their incorporation into the framework.
Determination of useful metal-organic frameworks was performed with thermogravimetric analysis (TGA) and powder X-ray diffraction (PXRD). In TGA, the mass of the sample is measured over time as it is heated. For a typical MOF, the weight will decrease by 15-20% and plateau for a few hundred degrees before completely breaking down, indicated a porous structure with an evaporable solvent trapped inside. In PXRD, the particle is pulverized and the intensity of Bragg reflection in the particulate is analyzed with a rotating X-ray source. For reasons that were not explained to me when I was 16 and which I don't plan on finding out now, peaks below 10° indicate a good crystal structure.
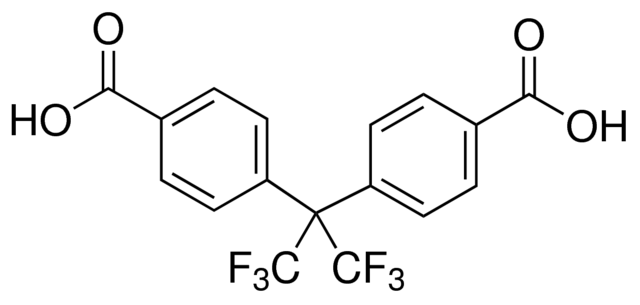
My final "product" was Mn(HFIPBB)(DMF). HFIPBB stands for 4,4'-(Hexaflouroisopropylidene)bis(benzoic acid), a bent ligand with two benzoic carboxylic acid sites and two CF3 groups. This was the first time this specific compound was synthesized, though an isostructural compound using cadmium was produced in [1]. Technically, the chemical formula is Mn(HFIPBB)(DMF)·DMF0.5, as the solvent also was adsorbed into the pores.
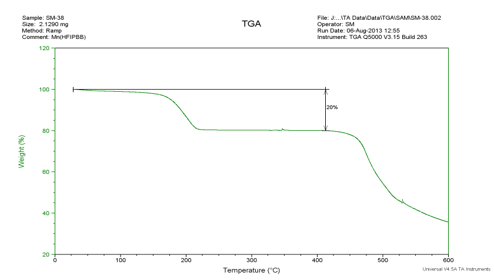
The TGA for this crystal showed a reasonable drop of 20-23% in mass and a plateau up to 400°C, indicating a porous crystal.
In addition, the PXRD for crystals grown using any of DMF, DMA, or DEF all showed the characteristic peaks below 10 degrees that indicate utility.
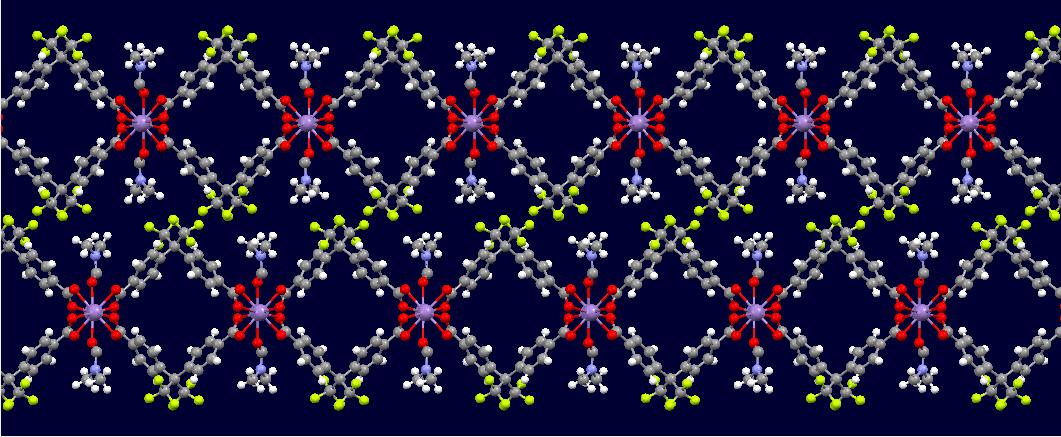
The image above shows this compound's structure, with long chains of manganese linked by two HFIPBB molecules and two DMF molecules filling in the gaps. The conformation is locked by the hydrophobic CF3 side chains, rather than by a chemical bond, which makes the crystal very unstable in response to external solvents. Attempts to link these chains with a bivalent solvent like TED (triethylenediamine) in place of DMF proved fruitless because of the steric hindrance of the side chains. Under methanol exchange, the crystal broke down and became brittle, indicating a lack of stability and an insufficient capacity for storing solvents. Last I heard, a sample was being sent off for magnetic study due to its similarity with a known antiferromagnet [2], but that was in 2013...
[1] H.-L. Jiang, B. Liu, and Q. Xu, "Rational Assembly of d10 Metal-Organic Frameworks with Helical Nanochannels Based on Flexible V-Shaped Ligand," Crystal Growth & Design 10 no. 2, (Feb, 2010) 806-811. [2] T. Yuen, C. L. Lin, L. Pan, X. Huang, and J. Li, "Magnetic Properties of a Metal-Organic Antiferromagnet Mn(hfipbb)py(H2O)0.5," Journal of Applied Physics 99 no. 8, (2006) 08J501.