Chapter 23
Chapter 23 Problem 18
Chapter 24 Problem 9, 10
a) Find the entropy
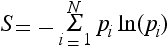 of the
system.
of the
system. b) The system undergoes a change which causes the probability of one microstate to increase by a small amount Δp1 , while another microstate decreases in probability by amount Δp2. Show that a first order Taylor series for ΔS predicts that the entropy will not change.
c) Show that a second order Taylor series for ΔS predicts that the entropy will decrease.
d) What could you do to an ideal gas that would make the velocity distribution more closely resemble the "fair" macrostate?
a) What are the pressure and temperature of the gas in its initial state? (Ignore the variation of pressure due to depth in the gas.)
b) In an experiment, the top half of the block is slowly removed (one thin layer at a time) and the reaction of the gas is observed. Find the pressure and temperature of the gas as a function of z, the height of the block. Assume the mass removal is slow enough that the block has negligible speed at all times.
c) In another experiment, the top half of the block is quickly removed and the reaction of the gas is observed. Find the pressure and temperature of the gas as a function of z, the height of the block.
d) For each experiment (part b and part c), what is the greatest height reached by the block? To solve one of these equations, you will have to use graphical methods or some trial-and-error on your calculator.
a) Show that for a general degree of freedom u (with energy E = a u2 ), the expectation value of the energy per particle is given by <E> = (1/2)kT , regardless of what the degree of freedom is. Hint: You must integrate over all values of the degree of freedom itself, not the energy.
b) Based on this theorem, what is the heat capacity per particle in a solid lattice? Look in HRK to verify this prediction.
c) The Equipartition Theorem works well in classical physics but not quantum systems. Imagine a system with only two possible energy levels, +E0 and -E0 (rather than a continuum). Again using Boltzmann statistics, calculate <E> per particle.
d) Find the heat capacity of this quantum system per particle.
a) Find the distribution function f(r) for the distance of particles from the origin.
b) Find the expectation values <x>, <x2>, <r>, and <r2>. Hint: you can avoid doing the integrals for some of these by using other arguments.
c) Find the heat capacity CV for this system.
a) Find the speed distribution function for the particles leaving the hole. Hint: are all particles in the box equally likely to hit the hole in a given time?
b) Show that the average kinetic energy of particles leaving the hole is 2kT.