Site Index
- Home
-
Research - Background Information
-
Research - Mobility of Taxol in Tight Microtubule Bundles
-
Research - Diffusion of Macromolecules Inside Microtubules
-
Research - Tau Induces Cooperative Taxol Binding to Microtubules
-
Publications and Presentations
- Classes
Links
Research
Background: What are Microtubules?
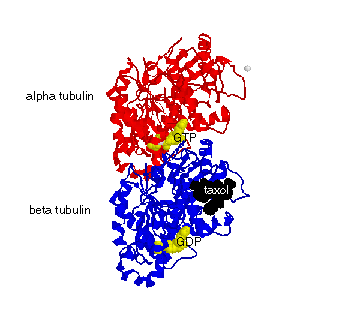
Microtubules are long, straight cylindrical filaments made from tubulin heterodimers (dimers). Each dimer contains an alpha and a beta subunit (see figure below). Each dimer binds GTP at the "nonexchangable site" (N-site), a pocket between the alpha and beta tubulin subunits. Another nucleotide pocket, the "exchangeable site" (E-site) binds GTP, but the nucleotide can be hydrolyded to form GDP. Dimers self assemble head-to-tail to form protofilaments, and protofilaments aggregate side-by-side to close into a cylindrical microtubule. Microtubules are found in all eucaryote cells and are used for structural support, movement, and active transport conduits.
Microtubules are 25 nm wide in outter diameter and 16nm across the inner diameter. Single microtubules are barely visible using Video-Enhanced Differential Interference Contrast (VE-DIC) light microscopy. In our lab, we use DIC and epi-fluorescence to visualize single microtubules and microtubule bundles. We also use Transmission Electron Microscopy (TEM) on negatively stained microtubules to see them at 200,000x normal size (see figure below).
| FIGURE: Tubulin dimer structure with GTP and GDP at the E-Site and N-site, respectively (yellow) and taxol (black). Tubulin dimers bind to each other head-to-tail to form linear protofilaments. These protofilaments bind side-to-side and curve around to form a hollow cylinder that is the microtubule. |
|
| FIGURE: Cartoon of a microtubule showing the relative dimensions of the protofilaments, dimers, and lumen (interior). |

|
| FIGURE: Transmission Electron Microscopy (TEM) on a negative stained microtubule. The protofilaments run long-wise along the microtubule length, but have a slight twist which causes a moire pattern to emerge, as can be seen here. |

|
Dimers have many binding sites on their surfaces. They bind GTP and GDP at the N and E-sites. They bind to each other longitudinally to make protofilaments, and they bind to each other laterally to make and close the microtubule. Microtubules bind motor proteins, such as kinesin, that can walk along the microtubules to carry cargo anywhere in the cell. Other microtubule-associated proteins (MAPs), like tau, bind to the microtubule exterior to strengthen, stiffen, and buttress the microtubule lattice. Theraputic drugs, such as taxol, are small enough to diffuse into and bind on the interior surface of the microtubule (see figure above). I have completed two studies on the biochemistry of taxol binding to microtubules (see Publications for abstracts on these studies).
Taxol is an chemotherapy drug used to fight cancer. Taxol works by stabilizing the microtubules of a cancer cell (tumor), so that they cannot fall apart. If the microtubules do not fall apart, the cell cannot divide. Eventually the cancer cell will grow too big for its own good because it cannot take in enough nutrients through its cell membrane to support its massive size. This causes the cancer cell to die because it cannot divide.
 Background Information - What's a Microtubule?
Background Information - What's a Microtubule?
 Mobility of Taxol in Tight Microtubule Bundles
Mobility of Taxol in Tight Microtubule Bundles
 Macromolecular Diffusion Inside and In Between Microtubules
Macromolecular Diffusion Inside and In Between Microtubules
 How Tau Affects Taxol Mobility
How Tau Affects Taxol Mobility