Project: Seeded peptide aggregation
Relevant paper: Morriss-Andrews, A, Bellesia, G, and Shea, J-E. "β-sheet propensity controls the kinetic pathways and morphologies of seeded peptide aggregation" J. Chem. Phys. 137, 145104 (2012)
We initialize this system with a pre-formed aggregate (a fibril, an amorphous oligomer, or a beta-barrel). Then we randomly distribute peptides in the bulk which can bind to the seed. Seeding aggregation with compatible existing aggregates is known to remove the nucleation barrier for aggregation. A fibrillar seed is shown in the image below.
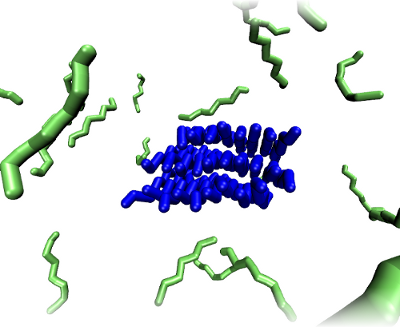
and the free peptides are shown in green.
We find the nature of both the aggregating peptides and aggregate seed affect the aggregation pathway and final aggregate structure. Fibril-prone free peptides template directly onto a fibrillar seed, where amorphous-prone free peptides can disrupt a fibrillar seed, causing an amorphous bubble of both free and seed peptides. In the fibril-prone case, the seed fibril does not lose its structure, so there is little mixing between free and seed peptides.
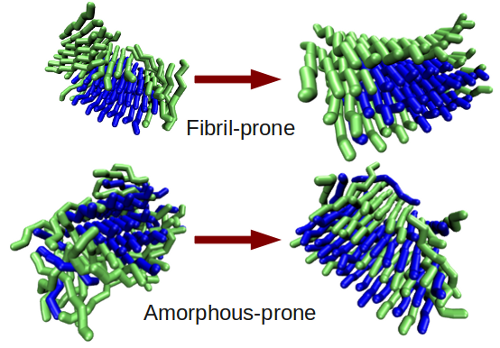
Our simulations suggest it is possible to sequester highly flexible peptides, prone to forming amorphous aggregates, by incorporating them into stable fibrillar aggregates, if such an aggregation-compatible seed can be found. This is significant, since amorphous oligomers are frequently found to have a higher degree of cytotoxicity, or toxicity to the cell.