 |
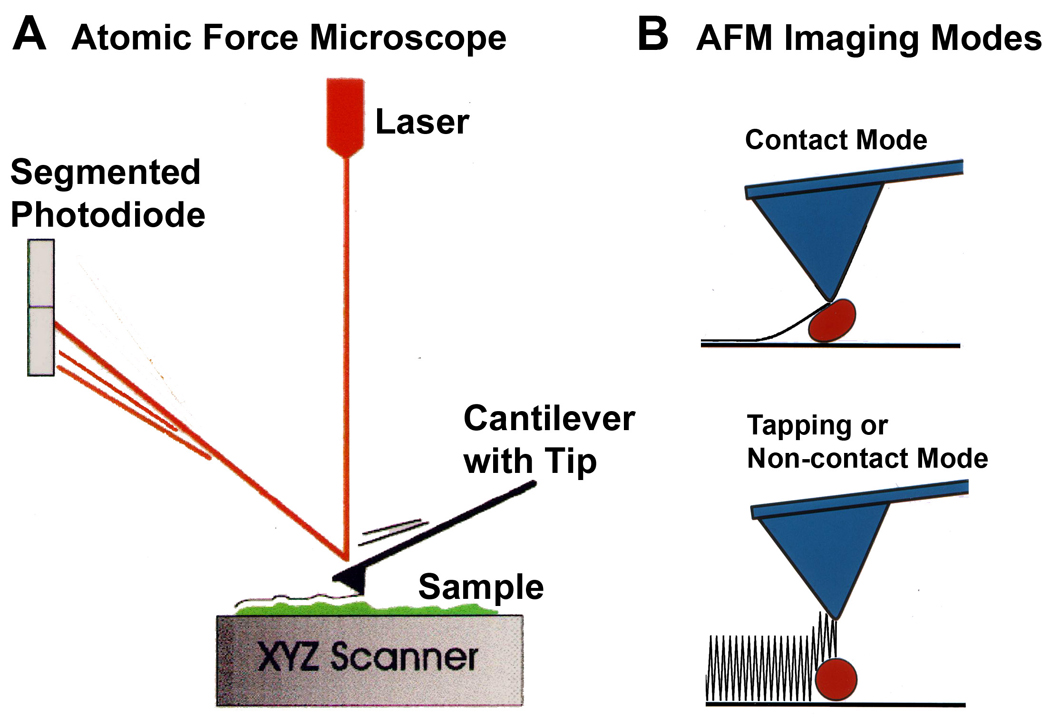
How does the AFM work? A. The atomic force microscope feels the surface of a sample with such a discriminating touch that it can sometimes even sense the individual atoms on the surface of a crystal such as gold. The AFM does this by raster-scanning a small tip back and forth over the sample surface. The tip is on the end of a cantilever, which deflects when the tip encounters features on the sample surface. This deflection is sensed with an optical lever (red line): a laser beam reflecting off the end of the cantilever onto a segmented photodiode magnifies small cantilever deflections into large changes in the relative intensity of the laser light on the two segments of the photodiode. In this way, the AFM makes a topographic map of the sample surface. B. AFM imaging in contact mode can damage or distort some soft biological samples. Tapping Mode or Non-contact Mode minimizes this problem by having the tip oscillate over the sample, making only brief intermittent contacts. This mode also provides additional information about the sample surface in the phase image that corresponds to the height image. |
| AFM of DNA single-stranded RNA, triple-stranded DNA and helix turns on double-stranded DNA | 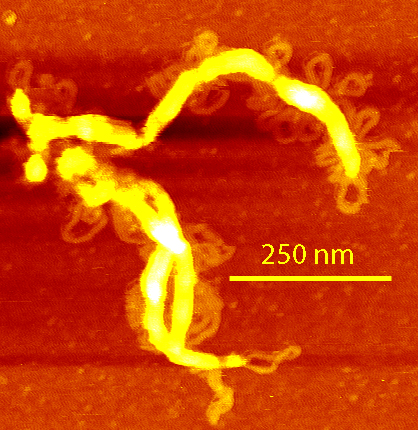 |
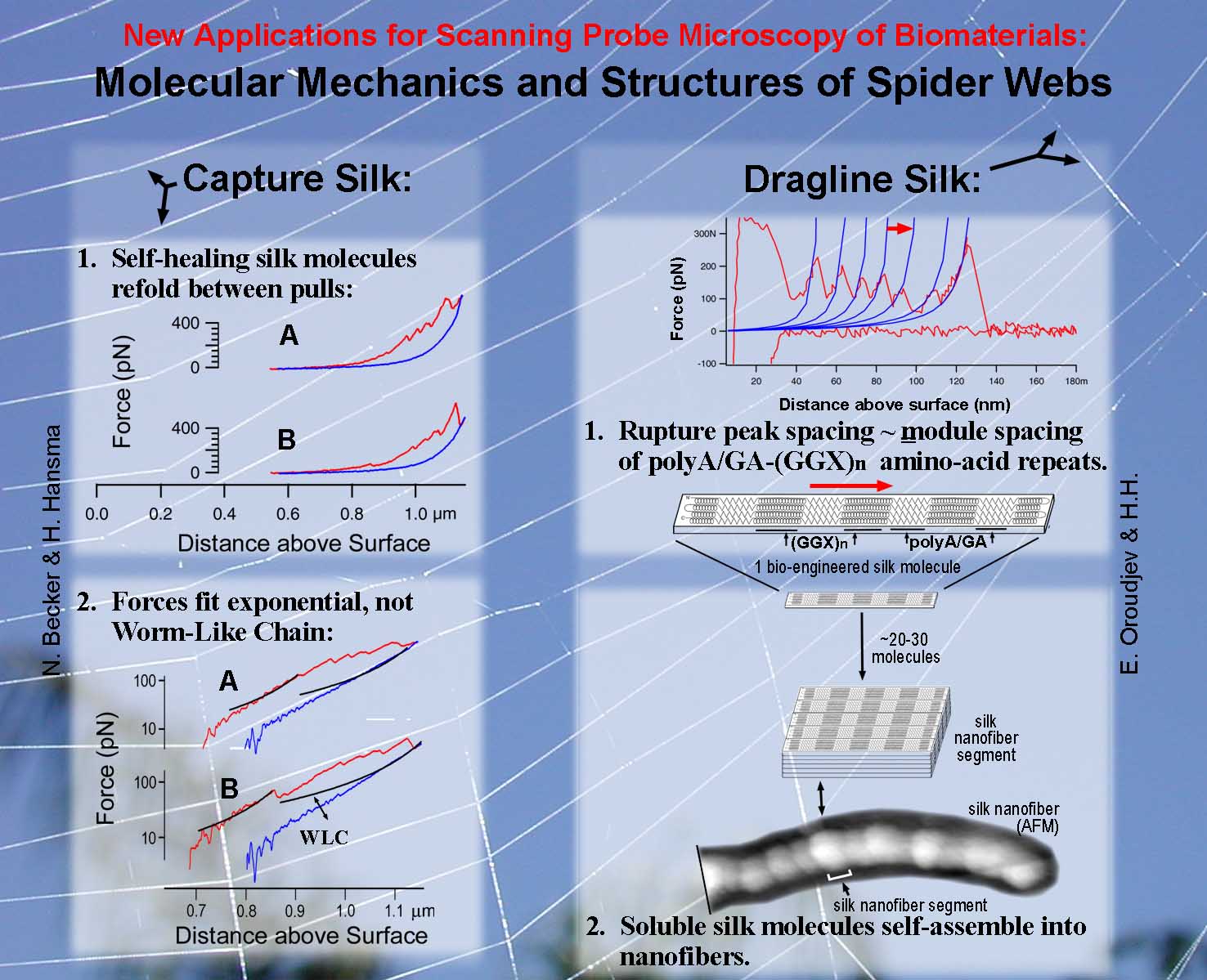 |
AFM and Force Spectroscopy of Spider Silk Left: Native spider capture silk showed exponential force increases when stretched. This has been modeled as a network of springs. See: Becker, N., Oroudjev, E., Mutz, S., Thompson, J. B., Cleveland, J., Proksch, R., Hansma, P. K., Hayashi, C., Makarov, D. E., and Hansma, H., Molecular Nanosprings in Spider Capture-Silk Threads, Nature Materials, 2, 278-283 (2003). PDF Right: Constructs of spider dragline silk formed segmented nanofibers See: Oroudjev, E., Soares, J., Arcidiacono, S., J. B. Thompson, Fossey, S. A., and Hansma, H. G., Segmented Nanofibers of Spider Dragline Silk: Atomic Force Microscopy and Single-Molecule Force Spectroscopy, Proc. Natl. Acad. Sci. USA, 99, 6460 (2002) |
| AFM of Basement Membrane Macromolecules Laminin-1 in air (right) and fluid showed a diversity of cross-shaped structures and dynamic conformational changes in fluid. See: Chen, C. H., Clegg, D. O., and Hansma, H. G., Structures and Dynamic Motion of Laminin-1 As Observed by Atomic Force Microscopy, Biochem., 37, 8262-8267 (1998). PDF Thread-like strands of Heparin Sulfate Proteoglycan (HSPG) were visualized without fixation or staining, and patterns of HSPG aggregation were observed. Positions of Laminin-1 on Collagen IV were mapped and compared with the positions of imperfections in the amino acid sequence of collagen IV. Molecular weights were estimated from the molecular volumes, as measured from AFM images. See: Chen, C. H., and Hansma, H. G., Basement Membrane Macromolecules: Insights from Atomic Force Microscopy, J. Struct. Biol., 131, 44-55 (2000). PDF | 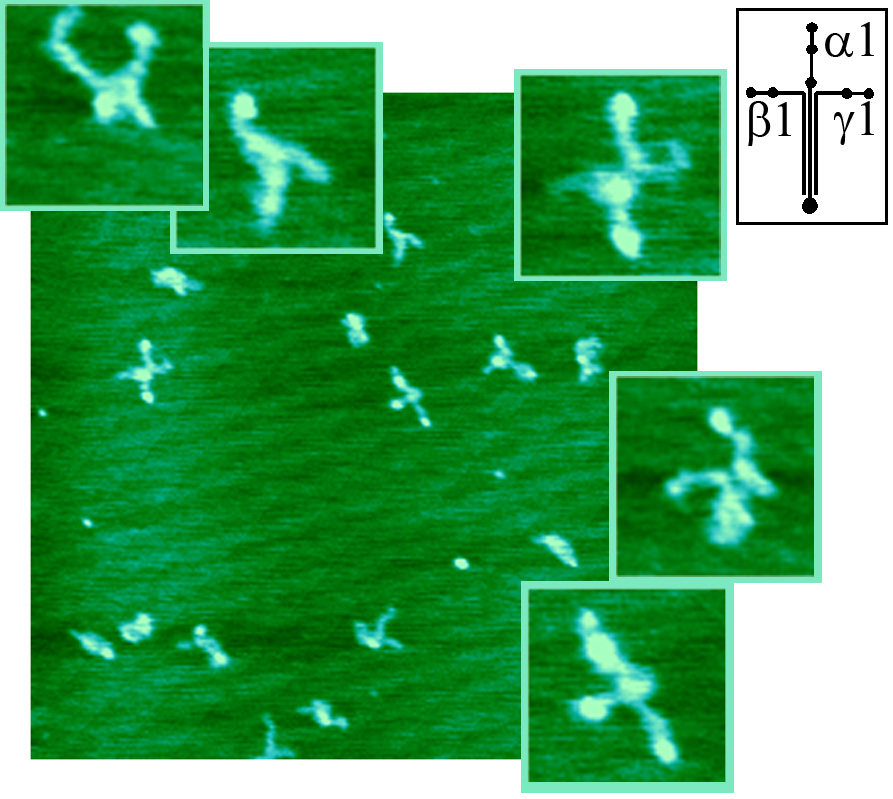 |
____________________________________
Helen Greenwood Hansma, PhD
Research Biophysicist Emeritus and Associate Adjunct Professor Emeritus
Department of Physics, University of California at Santa Barbara
Thank You  National Science Foundation
for supporting the AFM research!
National Science Foundation
for supporting the AFM research!